Electron Configuration Of Fe3. An fe3+ ion has five 3d electrons. Since 1s can only hold two electrons the next 2 electrons for iron go in the 2s orbital.
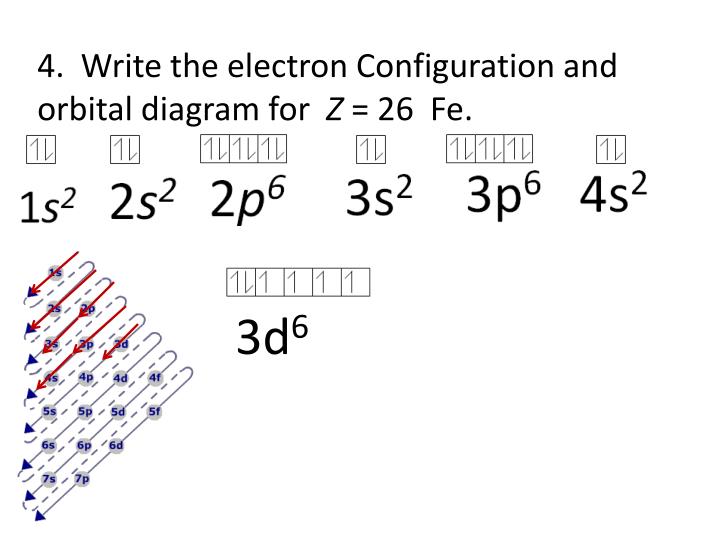
The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. In writing the electron configuration for chromium the first two electrons will go in the 1s orbital. Your email address will not be published.
Electron Configuration Of Oxygen Chlorine Electronic Configuration Chlorine Has An Atomic Number Of 17.
This is the order in which electrons are placed in the different electron shells and their sublevels. Secondly, what is fe2+ and fe3 +? There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configuration for fe3+ 1.3k views arya wagle , studied at pace junior science college, andheri answered 3 years ago
View The Full Answer Transcribed Image Text:
Difference about fe2+and fe3+ is the number of electrons, which in turn results in different properties. How many valence electrons does fe2+ have? The p orbital can hold up to six electrons.
There Are Two Main Exceptions To Electron Configuration:
For fe to convert to fe3+ it will have to lose 2 electrons from 4s orbital and 1 electron from 3d orbital. The electrons present in ballenced shell is […] Give the electronic configuration for fe3+.
Fe2+ Contains 2 Fewer Electrons Compared To The Electronic Configuration Of Fe.
The electron configuration of fe3 is ar 3d5. How many unpaired electrons are present? There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for fe3+.
Electron Configuration For Fe, Fe2+, And Fe3+ (Iron And Iron Ions) In Writing The Electron Configuration For Iron The First Two Electrons Will Go In The 1S Orbital.
Using the electron configuration for a neutral atom of iron, we can begin subtracting electrons from its outermost shell first. 1s2 2s2 2p6 3s2 3p6 3d5. What is the electron configuration for fe2+?